Towards Sustainable Mining: Challenges and Solutions for Lithium and Critical Metal Recovery
The Problem:
Towards the green transition: is the cure worse than the disease?
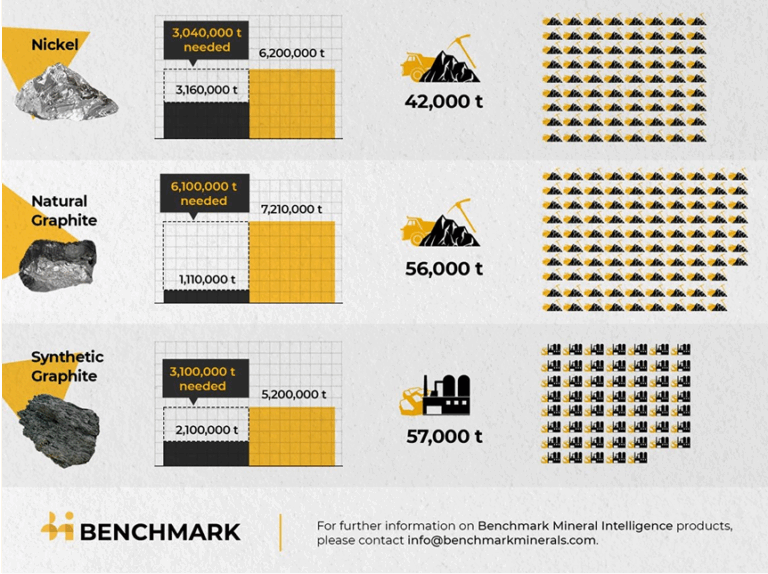
Lithium (Li), in addition to cobalt (Co) and nickel (Ni), is the key element of rechargeable electric car batteries and is critical for the global energy transition. Global annual Li consumption rate is expected to increase exponentially and reach 5.11 million tons by 2050 1. The EU´s Green Deal plan is forcing a complete shift to electric vehicles by 2035. Yet, Li rock mining causes irreversible environmental damage and destruction of entire ecosystems. Li processing from salt lake brines wastes enormous amounts of water. In Chile, Li mining has already depleted vital water supplies 2. Another critical raw material (CRM), Co, is mainly imported from Congo mines, plagued by child labour 3. Co end-of-life recycling input rate in the EU is only 22% 4. Furthermore, although not listed as critical, Ni is sourced from politically unstable countries. The ongoing Ukraine-Russia war has skyrocketed the price of Ni in March 2022, and revealed our vulnerability caused by the lack of CRMs.
The World Bank estimates that over 3 billion tons of minerals and metals (Cu, Zn, Pb, etc) are needed to deploy wind, solar and geothermal power, and energy storage, to reach a below 2ºC future.
The Opportunity:
Chasing the circular economy: the promise of urban mining.
To minimize the environmental damage of Li mining and secure a stable supply of Li and valuable metals the industry has to minimize its reliance on the traditional linear supply chain and introduce energy-efficient and sustainable technologies for metal recovery from complex streams.
The opportunities for reinventing Li mining are plentiful; lithium ion battery (LIB) recycling, which currently recovers Ni and Co only partially and does not recover Li or has low Li recovery, discharges wastewater with 1-3 g L-1 of Li 5. Li is also present in reverse osmosis (RO) desalination brines (5-7 mg L-1 Li) 6, as well as geothermal brines (10-50 mg L-1 Li)7. There are 18 geothermal power plants in the EU where geothermal brines, enriched with Li by subsurface water-rock interactions, contain between 63-480 mg L-1 of Li 8, and represent an untapped Li resource in Europe. Besides Li, industrial wastewaters – acid mine drainage, produced waters from oil/gas recovery, electroplating wastewater, e-waste leachate, represent secondary sources of Co, Ni, Cu, Pd, Pt, etc. Yet, their selective recovery using existing methods (adsorption, ion exchange, precipitation, membrane separation, coagulation-flocculation, electrowining) is very challenging due to the extreme heterogeneity, high salinity, and a variety of organic pollutants present.
The Solution:
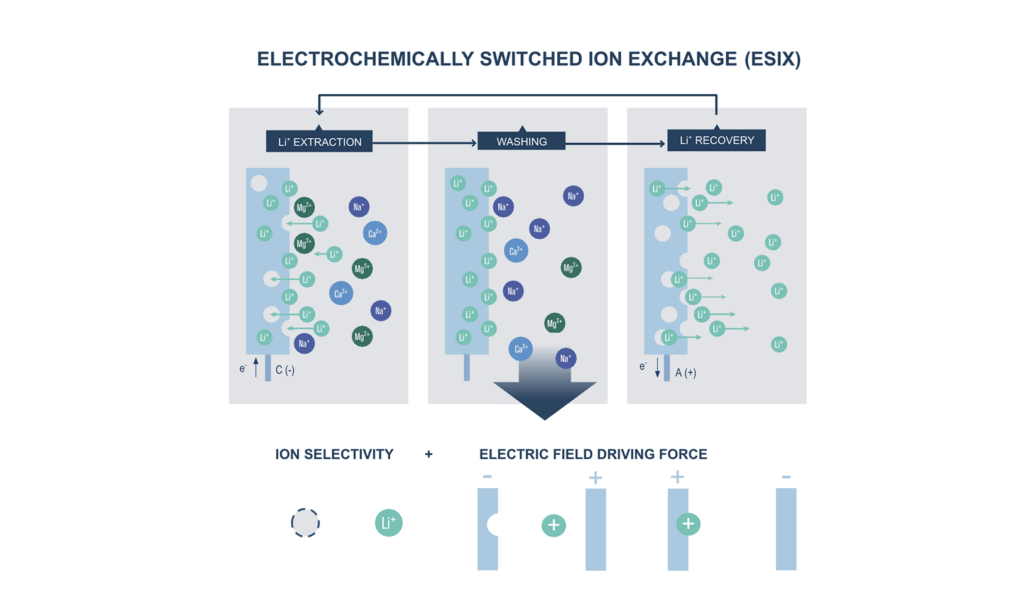
Electrochemically switched ion exchange (ESIX) is a three-step process: 1) Li+/Mn+ is inserted into the ion-selective, negatively polarized electrode (analogous to battery discharge), 2) source water is replaced with a recovery solution, and 3) captured Li+/Mn+ is transferred into the recovery solution by polarity reversal (analogous to battery charging). This cycle is repeated until the recovery solution reaches the desired Li/M concentration. Yet, reaching the concentration and purity required for the industrial exploitation of metal concentrate is difficult using existing electrodes, mainly derived from the battery field.
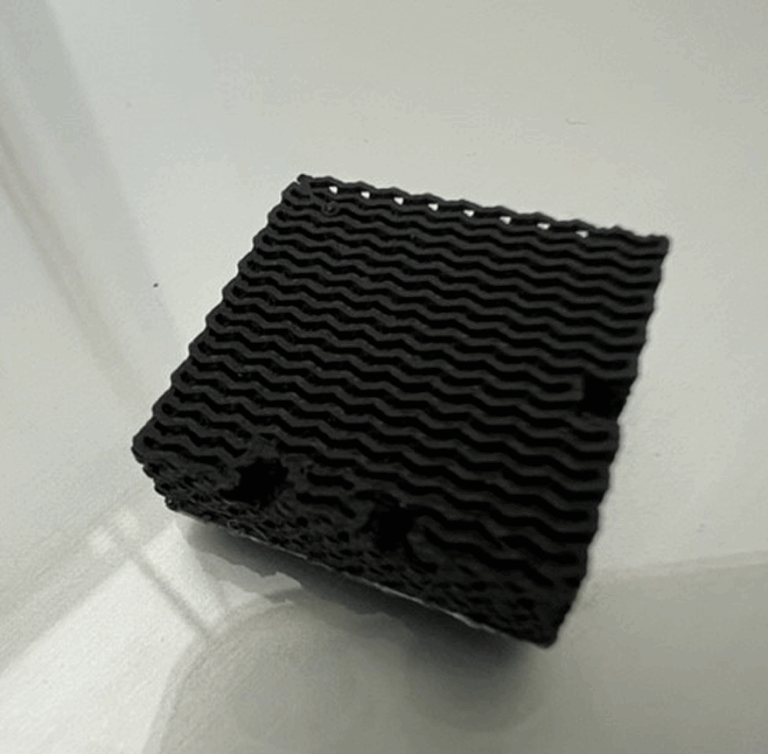
ELECTROmonoLITH project aims to address several challenges of the existing ESIX-based processes – e.g., poor material stability of conventional, battery-derived electrodes in complex waste streams, low recovery capacity and rate, insufficient selectivity and high overall energy consumption, by developing new, monolith electrodes with mass transfer-enhancing structure, tailored for highly selective capture of specific metals from multi-component solutions using surface-imprinted ion-selective recognition units.
Highly ordered electrode architectures with accessible active sites for metal ion cycling will endow the monolith electrodes with high space-time yields, minimize the pressure drop and thus hydraulic energy requirements, and enable the production of large volumes of high purity CRM concentrates in a more energy-efficient way. Given the inherent modularity of electrochemical systems, their scalability, autonomous operation and easy coupling to renewable energy sources, as well as the possibility to integrate electro-extraction of target metals with electrooxidation of organic pollutants, ELECTROmonoLITH project has the potential to provide a platform technology for both resource recovery and wastewater treatment, and to respond to major challenges of the water-energy nexus
- Ralon, P.; Taylor, M.; Ilas, A.; Diaz-Bone, H.; Kairies, K. Electricity Storage and Renewables: Costs and Markets to 2030, International Renewable Energy Agency, Abu Dhabi, UAE.; 2017.
- https://www.ocmal.org/lithium-firms-depleting-vital-water-supplies-in-chile-analysis-suggests/. 2019.
- Commission, E., Study on the EU’s List of Critical Raw Materials. 2020.
- Commission, E., Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability. 2020.
- Kim, S.; Kim, J.; Kim, S.; Lee, J.; Yoon, J., Electrochemical lithium recovery and organic pollutant removal from industrial wastewater of a battery recycling plant. Environmental Science: Water Research & Technology 2018, 4 (2), 175-182.
- Kim, S.; Joo, H.; Moon, T.; Kim, S.-H.; Yoon, J., Rapid and selective lithium recovery from desalination brine using an electrochemical system. Environmental Science: Processes & Impacts 2019, 21 (4), 667-676.
- Lin, H.; Yu, X.; Li, M.; Duo, J.; Guo, Y.; Deng, T., Synthesis of Polyporous Ion-Sieve and Its Application for Selective Recovery of Lithium from Geothermal Water. ACS Applied Materials & Interfaces 2019, 11 (29), 26364-26372.
- Stringfellow, W. T.; Dobson, P. F., Technology for the Recovery of Lithium from Geothermal Brines. Energies 2021, 14 (20), 6805.